| McSwiggen & Associates |
| /Tech Notes/Qualitative Analysis |

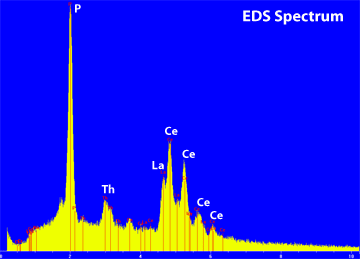
The term "Qualitative Analysis" refers to the process of determining which elements are present in a sample. Two different types of spectrometers can be used, a wavelength dispersive spectrometer (WDS) and an energy dispersive spectrometer (EDS) (see WDS vs EDS). Each has its advantages and disadvantages. However for qualitative analysis, it is a matter of speed versus energy resolution. An EDS spectrum can be collected in a very short period of time, but it has very poor energy resolution. As a result, there can be very serious overlaps between the various X-ray lines. This can be seen in the example below. Both the WDS spectrum and the EDS spectrum were collected on a monazite grain (Ce, La, Nd, Th)PO4. The portion of the WDS spectrum displayed covers the energy range from about 4.31 keV to 7.18 keV, the range where X-ray lines for the rare earth elements occur. Even in this extremely complicated phase, the X-ray lines are spread out enough to identify the individual lines. |
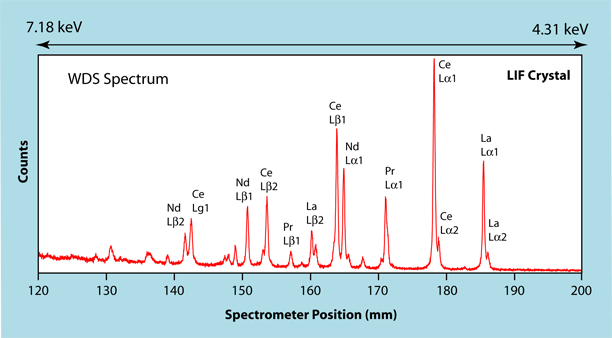
In contrast, in the EDS spectrum shown at the left, the X-ray lines for the rare earth elements (Ce & La) completely overlap on each other. This makes it impossible to determine if there are any other rare earth elements present. In the WDS spectrum one can clearly see that in addition to Ce and La, Pr and Nd are also present in the sample. For quick phase identifications, typically EDS spectra are used, because the process is faster. However if the objective is to determine all of the elements present, then only WDS spectra should be used because of the superior energy resolution. |